What is CoolSculpting Mini?
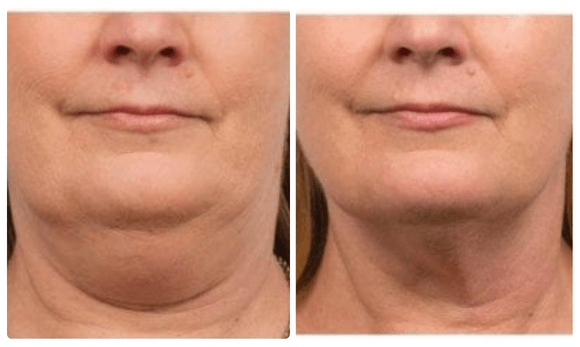
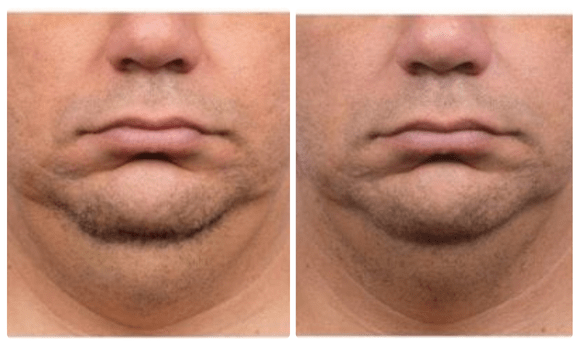
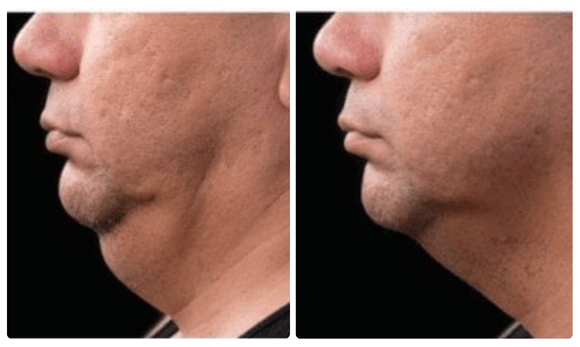
CoolSculpting which is FDA approved to treat submental fat, or double chin, with their clinically proven technology that freezes unwanted fat. The treatment allows the body to naturally eliminate the fat over a 12 week period.
FAQs
How long do treatments take?
The new applicator time is 45 minutes. Average total treatment visit time is around one hour.
Is there downtime?
There typically is no downtime with this procedure, you can resume your normal activities immediately.
When will I see my results?
Typically, it will be seen in as early as 3 weeks with optimal results being seen between 3-4 months.
Before and After

Your Best Skin Awaits
Visit North Atlanta Dermatology
Our team provides thoughtful, expert care for all your skin health needs. We are proud to offer the most advanced medical, surgical, and cosmetic dermatologic services in the North Atlanta region.

